The chemiluminescence principle
Chemiluminescence refers to light which is emitted after excitation of a molecule during a chemical reaction. It is being used in the analysis of nitrogen gases.
Nitrogen monoxide reacts with ozone to excited nitrogen dioxide.
The subsequently emitted light (proportional to the NO-concentration) is measured and amplified by a photomultiplier.
In order to analyse higher nitrogen oxides, they have to be reduced with a catalyst (NOX-converter) to nitrogen monoxide.
NO + O3 → NO2* + O2
NO2* → NO2 + hν (luminescence)
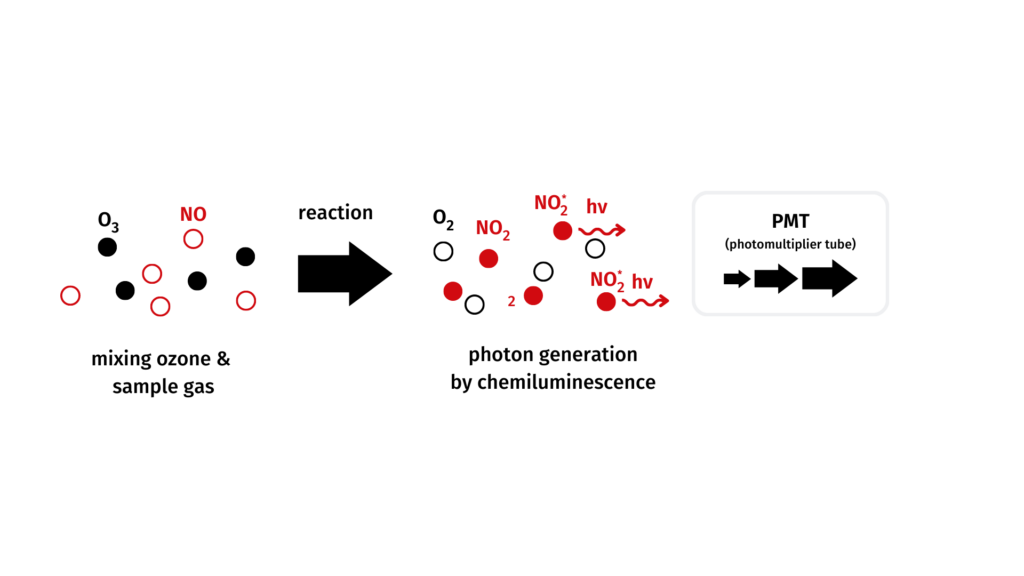
Measuring principle of CLDmono
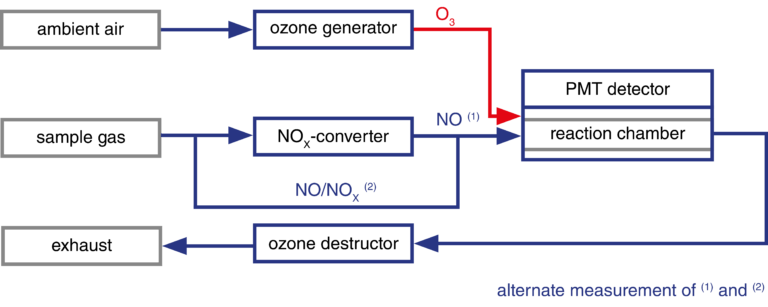
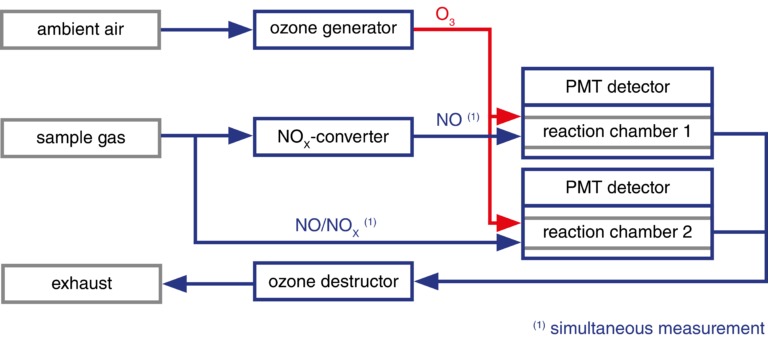
With the CLDmono, the sample gas can be alternately passed through and past the NOX converter. This means that NO and NOX can be measured alternately with one reaction chamber (measuring chamber). With the CLDdual, a second measuring chamber enables simultaneous measurement of the two gases.
Measuring principle of CLDdual
Direct measuring method
Extremely stable measurement
High dynamic range
Extremely low maintenance
Need help?
We look forward to help you choose the perfect gas analysis products for your needs. Talk to us about different technologies and our CLD analyzers & OEM sensors.
