Flame Ionization Detector
For the measurement of: Hydrocarbon compounds (THC) and methane (CH4)
FID - Flame Ionization Detector
Flame Ionization Detection is used to measure organic compounds and is used in particular for the detection of hydrocarbon compounds (HC). The measurement principle is based on the ionization of the carbon atoms contained in the sample gas in a hydrogen flame. Mixing the hydrogen with clean combustion air produces oxyhydrogen gas, which ignites in the combustion chamber when the glow plug is ignited. The flame burns within the electric field between two electrodes, producing CH+ and H3O- ions by chemical ionization. These are drawn from the DC voltage applied to the electrodes. Depending on the number of HC compounds contained in the sample gas or the number of organically bound carbon atoms (C atoms), a corresponding current is obtained at the electrodes. This current is very low (pA range) and is therefore electrically amplified for detection. The hydrocarbon concentration in the sample gas can be inferred from this current due to the linear proportionality between the quantities.It is a highly accurate, stable and a direct measurement (independent of background gases).
It is based on the fact that light is absorbed by gas molecules. In the photoacoustic spectroscopy the modulated light is converted into acoustic waves. The resulting soundwaves are converted into an electric signal with the use of a microphone.
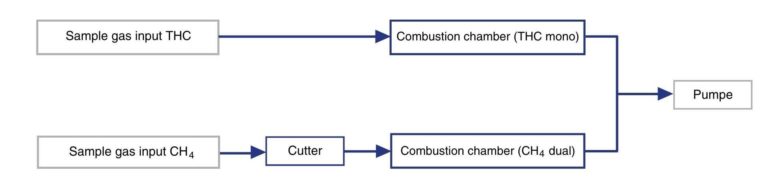
In dual measurement, methane (CH4) is measured in a second combustion chamber. For this purpose, long-chain atoms are reduced with the aid of a thermal cutter so that only methane remains in the sample gas for measurement. The concentration of non-methane hydrocarbons (NMHC) is then determined from the difference.
Since this measurement principle mainly ionizes the carbon atoms (C atoms), higher compounds with several C atoms (e.g. C3H8) also give a higher current at the detector. If the proportion of the various HC compounds is to be specified, this can be determined by means of so-called response factors. These are strongly dependent on the design of the measuring chamber and the flow rate of sample gas, which is why response factors are determined individually for each flame ionization detector.
Measuring principle - combustion chamber structure
A mixture of hydrogen (combustion gas) and the sample gas to be analyzed flows into the combustion chamber. By supplying the combustion air, an oxyhydrogen flame can be ignited with the aid of the glow plug.
The electrodes for detecting the current caused by the ionization are arranged in a shell around the flame. An electrical amplification of the very low currents takes place before the detector. Both the combustion air (synthetic air) and the combustion gas (H2) must be supplied externally to the FID.

Technology factsheet
We have summarized the most important information about the technology, possible applications and our technical design options in a clear factsheet.
FLame ionization detection
Benefits at a glance
Very high dynamic range
Extremely high linearity over wide concentration range
Simultaneous measurement of THC and CH4 using cutter technology
Continuous measurement
Suitable for continuous operation
Need help?
We look forward to help you choose the perfect gas analysis products for your needs. Talk to us about different technologies and our customer-specific FID gas analyzers or OEM sensors.
